Le et al. recently reported (Science 10.1126/science.aan6733 (2017); (ClinicalTrials.gov number, NCT01876511) on the success of immune checkpoint blockade with anti-PD-1 antibodies in the patients whose cancers exhibited microsatellite instability-high (MSI-H) status. The study included 12 different histologic cancer subtypes (n=86) with proved mismatch repair-deficiency status assessed by either polymerase chain reaction or immunohistochemistry. Initial results indicate that objective radiographic responses were present in 53% of patients while complete responses were achieved in 21% of patients.
The study was based on the previous preclinical and clinical data that revealed the propensity of mismatch-repair deficient (MMR) cancers to harbor a large number of somatic mutations resulting in increased load of mutation-associated neoantigens recognized by the immune system.
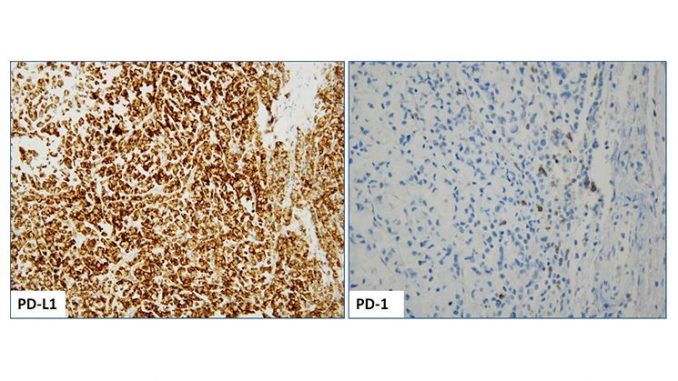
Based on this and other data, on May 23, 2017, the U.S. Food and Drug Administration (FDA) granted accelerated approval to anti-PD-L1 drug pembrolizumab (KEYTRUDA®, Merck & Co.) for adult and pediatric patients with unresectable/metastatic MSI-H/MMR deficient solid tumors (regardless the histotype) that have progressed following prior treatment and without satisfactory alternative treatment modalities. The approval also covered MSI-H colorectal cancer patients who progressed following treatment with a cytotoxic therapy (Fluoropyrimidine, oxaliplatin, and irinotecan) (see illustrative figure with PD-1/PD-L1 positive sporadic MSI-H colorectal cancer).
These news revolutionize the cancer treatment paradigm as for the first time the cancer treatment is based solely on the molecular characteristics of cancer (in this case microsatellite instability/MSI/ status) ignoring completely tumor morphology (histotype of tumor). This appears to be “the FDA’s first tissue/site-agnostic approval”.
Semir Vranić, M.D., Ph.D.,
Editor-in-Chief, Bosnian Journal of Basic Medical Sciences
Leave a Reply