Targeted therapy based on the immune checkpoint inhibitors (Anti-PD1/PD-L1) has revolutionized cancer treatment and outcome of several solid and hematologic malignancies, including non-small cell lung carcinoma (NSCLC), metastatic renal cell carcinoma, metastatic malignant melanoma, advanced/metastatic urothelial carcinoma and refractory/relapsed classical Hodgkin lymphoma.
Many ongoing clinical trials (>400 available now at www.clinicaltrials.gov) clearly indicate that more approvals based on PD-L1 status will become available soon.
Recently, Food and Drug Administration (FDA) has approved avelumab (MSB0010718C, BAVENCIO, EMD Serono, Inc.) for the treatment of patients with metastatic Merkel cell carcinoma (it is a highly aggressive variant of skin neuroendocrine carcinoma). The approval pertains to the treatment of adult and pediatric patients (≥12 years) with the metastatic disease. Avelumab is anti-PD-L1 IgG1 monoclonal antibody that binds to PD-L1 inhibiting its interactions with PD-1 and consequently enables activation of T-cells and the adaptive immune system.
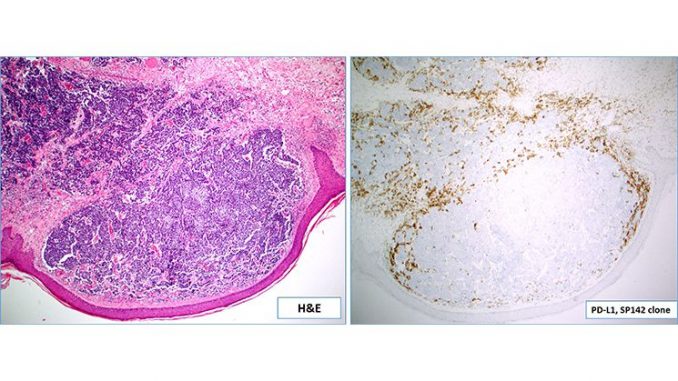
The approval was based on the results obtained in an open-label, single-arm, multi-center clinical trial (JAVELIN Merkel200 trial) that demonstrated a good overall response rate of 33% including 11% complete responses and 22% partial responses. Interestingly, PD-L1 status (expression) did not affect the response rate in the present study (See the Figure as an example of PD-L1 positive Merkel cell carcinoma). Previous approvals by FDA (NSCLC, bladder cancer) also included different antibodies and immunohistochemical platforms with different scoring criteria for selecting patients for immune checkpoint inhibitor therapies.
By: Semir Vranić, M.D., Ph.D., Editor-in-Chief, Bosnian Journal of Basic Medical Sciences, and Zoran Gatalica, M.D., D.Sc., Executive Medical Director, Caris Pathology, Caris Life Sciences, Phoenix, AZ, USA
Leave a Reply