Understanding Retinal Vein Occlusion (RVO)
Retinal Vein Occlusion (RVO) is a critical eye condition, second only to diabetic retinopathy in prevalence among retinal vascular diseases. It occurs when one of the veins in the retina becomes blocked, leading to potential vision loss or blindness. This blockage often results in complications such as neovascular glaucoma and macular edema. Traditional treatments focus on managing these complications, primarily through anti-vascular and anti-inflammatory medications, as well as retinal laser therapy. However, the complexity of these treatments limits their accessibility and effectiveness. RVO is more common in older individuals, typically those over the age of 50. Certain health issues, such as high blood pressure, diabetes, glaucoma, and high cholesterol, increase the risk. Conditions that affect blood clotting or circulation can also contribute to RVO. Additionally, other eye problems, like inflammation inside the eye, can increase the risk. Smoking and obesity are known to elevate the risk of RVO.
The symptoms of RVO can vary but typically include:
- Sudden and Painless Vision Loss: This is the most common symptom. The severity depends on the extent and location of the blockage.
- Blurred or Distorted Vision: Known as metamorphopsia, where straight lines appear wavy or bent.
- Floaters: Small spots or lines that float in the field of vision.
Regular eye exams, especially for those with risk factors, are essential for the early detection and effective management of RVO.
Treatment for RVO aims to manage the symptoms and prevent further complications. Options include:
- Medications: To reduce macular edema or treat neovascularization, such as anti-VEGF injections.
- Laser Therapy: To seal leaking blood vessels or reduce swelling in the retina.
- Control of Underlying Conditions: Managing diabetes, high blood pressure, and other related health issues is crucial.
The Intriguing Role of Neutrophil Extracellular Traps in RVO
Recent research has uncovered a potential link between Retinal Vein Occlusion (RVO) and Neutrophil Extracellular Traps (NETs), a relatively recently discovered element of the body’s immune response. NETs, web-like structures released by neutrophils (a type of white blood cell), play a crucial role in fighting infections. Composed of DNA fibers, histones, and various antimicrobial proteins, these structures contribute significantly to the formation of blood clots or thrombosis within the retinal veins in RVO.
Neutrophils, the most abundant white blood cells, undergo a unique form of cell death known as NETosis upon encountering pathogens. During NETosis, they release their nuclear contents, forming a sticky, web-like mesh. This mesh, known as Neutrophil Extracellular Traps (NETs), consists of decondensed chromatin (DNA and histones), serving as a scaffold. Histones within NETs play a crucial role in trapping and neutralizing pathogens. Proteins such as myeloperoxidase (MPO) and neutrophil elastase, embedded within these NETs, combat bacteria, fungi, and other pathogens. NETs primarily function to trap pathogens, containing their spread, and also modulate the immune response by interacting with other immune cells.
In cases of Retinal Vein Occlusion (RVO), NETs can exacerbate blockages in retinal veins by providing a structure for red blood cells and platelets to aggregate. This indicates that targeting NETs could be a promising approach in treating RVO. Additionally, excessive formation or inadequate degradation of NETs can lead to chronic inflammation, autoimmune diseases, and even cancer. Current research efforts are focused on understanding NETs to develop diagnostics and treatments for conditions where NETs are implicated.
Efforts are underway to create therapies that either inhibit NET formation or promote their degradation, thus addressing diseases associated with excessive NET formation. Neutrophil Extracellular Traps are vital in the innate immune response and in defending against infections. Their role in RVO and other pathologies highlights the complexity of the immune system and the necessity for a balanced immune response. Ongoing research into NETs is paving the way for new insights and treatments for a wide range of diseases.
Patients’ Profile and Methodology
In this study, 30 patients diagnosed with RVO were compared with 30 healthy individuals. The patients were carefully selected, ensuring they had no history of other thrombotic or systemic inflammatory diseases. The study analyzed various coagulation function parameters and NETs biomarkers, such as cell-free DNA (cf-DNA), myeloperoxidase-DNA (MPO-DNA), and neutrophil elastase (NE). These biomarkers are crucial indicators of NETs activity and can provide valuable insights into the relationship between NETs and RVO.
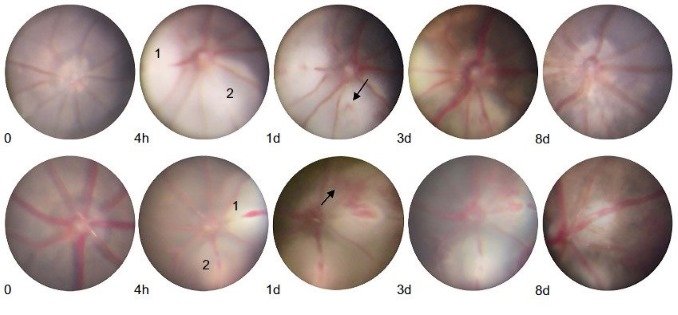
The senior author, Jun Zhang, from the Department of Ophthalmology, Changzhou Third People’s Hospital, Changzhou Medical Center, Nanjing Medical University, Changzhou, China, explained that the study also extended to animal models, where an RVO mouse model was created using retinal laser therapy. The mice were divided into groups, with some receiving DNase I injections and others serving as controls. This setup allowed for a detailed examination of how DNase I affects NETs formation and the progression of RVO. The analysis included retinal imaging, blood sample collection, and histological evaluations to assess the impact of DNase I on the retinal vessels.
DNase I: A Potent NETs Inhibitor
Deoxyribonuclease I (DNase I) emerges as a focal point in this research. This enzyme, known for its ability to degrade DNA, acts as a natural inhibitor of NETs. DNase I’s role in medical treatments has been underutilized despite its potential benefits, particularly in vascular diseases. In this study, DNase I was explored as a therapeutic agent to mitigate the effects of NETs in RVO. The hypothesis was that by breaking down the DNA in NETs, DNase I could prevent the formation of blood clots in the retina.
The results were interesting. Both human and mouse models showed increased levels of NETs biomarkers in cases of RVO. The administration of DNase I in the mouse model led to a significant reduction in these biomarkers. More importantly, DNase I shortened the duration of retinal thrombus in the RVO mice. These findings were corroborated by immunofluorescence images and Western blot analysis, which showed a decrease in NETs-related markers post-DNase I treatment.
The findings indicate that DNase I, by targeting Neutrophil Extracellular Traps (NETs), could potentially alleviate the symptoms and progression of Retinal Vein Occlusion (RVO). However, the study also recognizes certain limitations, such as the necessity for further research into the optimal dosage of DNase I and its specific mechanisms of action in the context of RVO. Additionally, the study highlights the importance of including larger and more diverse patient groups in future research to enhance the validity of the results.
The first author, Guohua Deng, from the Department of Ophthalmology, Changzhou Third People’s Hospital, Changzhou Medical Center, Nanjing Medical University, Changzhou, China, empahised that the study positions DNase I as a promising candidate for the treatment of RVO, particularly due to its effectiveness in reducing NETs formation and the duration of retinal thrombus. This represents a potential shift in the therapeutic approach to RVO, moving from merely managing complications to addressing one of the primary underlying causes of the condition. Further studies are crucial to thoroughly understand the role of DNase I and to establish a foundation for its integration into clinical treatments for RVO.
The translation of the preceding English text in Chinese:
理解视网膜静脉阻塞(RVO)
视网膜静脉阻塞(RVO)是一种严重的眼部疾病,在视网膜血管疾病中仅次于糖尿病视网膜病变。当视网膜中的一条静脉发生阻塞时,可能会导致视力丧失甚至失明。这种阻塞常常导致新生血管性青光眼和黄斑水肿等并发症。传统治疗着重于管理这些并发症,主要通过抗血管和抗炎药物治疗,以及视网膜激光疗法。然而,这些治疗的复杂性限制了它们的可及性和有效性。RVO在老年人中更为常见,通常发生在50岁以上的人群。诸如高血压、糖尿病、青光眼和高胆固醇等健康问题增加了患病风险。影响血液凝固或循环的条件也可能导致RVO。此外,眼内其他问题,如眼内炎症,也可能增加风险。吸烟和肥胖被认为会增加患RVO的风险。
RVO的症状可能有所不同,但通常包括:
– 突然而无痛的视力丧失:这是最常见的症状。严重程度取决于阻塞的范围和位置。
– 视力模糊或扭曲:被称为变形视,即直线看起来波浪形或弯曲。
– 飞蚊症:视野中飘浮的小斑点或线条。
定期进行眼部检查,尤其对于有风险因素的人来说,对于早期发现和有效管理RVO至关重要。
RVO的治疗目的是管理症状并预防进一步的并发症。治疗选项包括:
– 药物治疗:用于减轻黄斑水肿或治疗新生血管化,如抗VEGF注射。
– 激光疗法:用于封闭渗漏的血管或减少视网膜水肿。
– 控制潜在疾病:管理糖尿病、高血压和其他相关健康问题至关重要。
中性粒细胞胞外陷阱在RVO中的有趣作用
近期研究发现了视网膜静脉阻塞(RVO)与中性粒细胞胞外陷阱(NETs)之间的潜在联系,NETs是机体免疫应答中相对较新发现的元素。NETs是由中性粒细胞(一种白细胞)释放的网状结构,对于抵御感染发挥着关键作用。它们由DNA纤维、组蛋白和各种抗菌蛋白组成,对于RVO中视网膜静脉中血栓或血栓形成有重要贡献。
中性粒细胞是最丰富的白细胞,在遇到病原体时会经历一种称为
NETosis的独特细胞死亡形式。在NETosis过程中,它们释放核内物质,形成粘性的网状结构。这种网状结构,即中性粒细胞胞外陷阱(NETs),由解凝的染色质(DNA和组蛋白)构成,作为支架。NETs内的组蛋白在捕获和中和病原体中起到关键作用。嵌入在这些NETs中的蛋白质,如过氧化物酶(MPO)和中性粒细胞弹性酶,可以对抗细菌、真菌和其他病原体。NETs的主要功能是捕获病原体,控制其扩散,并通过与其他免疫细胞的相互作用来调节免疫应答。
在视网膜静脉阻塞(RVO)的情况下,NETs可以通过为红细胞和血小板提供聚集结构来加剧视网膜静脉的阻塞。这表明针对NETs可能是治疗RVO的一个有前景的方法。此外,NETs的过度形成或不充分降解可能导致慢性炎症、自身免疫性疾病乃至癌症。目前的研究努力集中于理解NETs,以发展用于诊断和治疗NETs相关疾病的方法。
目前正在开发旨在抑制NETs形成或促进其降解的疗法,以解决与NETs过度形成相关的疾病。中性粒细胞胞外陷阱在先天免疫应答中至关重要,在抵御感染中发挥作用。它们在RVO和其他病理中的作用凸显了免疫系统的复杂性和维持平衡免疫应答的必要性。持续对NETs的研究正为一系列疾病的新见解和治疗方法铺平道路。
病人特征和研究方法
在一项研究中,30名被诊断为RVO的患者与30名健康个体进行了比较。患者经过仔细挑选,确保他们没有其他血栓性或全身性炎症性疾病的病史。研究分析了各种凝血功能参数和NETs生物标志物,如细胞游离DNA(cf-DNA)、过氧化物酶-DNA(MPO-DNA)和中性粒细胞弹性酶(NE)。这些生物标志物是NETs活动的关键指标,可以提供关于NETs与RVO关系的宝贵见解。
该研究还扩展到动物模型,其中使用视网膜激光疗法创建了RVO小鼠模型。小鼠被分成不同组,一些接受了DNase I注射,另一些则作为对照组。这种设置允许详细检查DNase I如何影响NETs的形成和RVO的进展。分析包括视网膜成像、血液样本采集和组织学评估,以评估DNase I对视网膜血管的影响。
DNase I:强效NETs抑制剂
去氧核糖核酸酶I(DNase I)在这项研究中成为焦点。尽管DNase I以其降解DNA的能力而闻名,但尤其在血管疾病中,它作为NETs的天然抑制剂的潜在益处一直未被充分利用。在这项研究中,探讨了DNase I作为减轻RVO中NETs影响的治疗剂。假设是通过分解NETs中的DNA,DNase I可以防止视网膜中的血栓形成。
结果令人着迷。在RVO病例中,无论是人类还是小鼠模型,NETs生物标志物的水平都增加了。在小鼠模型中,DNase I的给药导致这些生物标志物显著减少。更重要的是,DNase I缩短了RVO小鼠的视网膜血栓持续时间。这些发现得到了免疫荧光图像和Western blot分析的证实,这些分析显示了DNase I治疗后与NETs相关的标志物的减少。
研究结果表明,通过针对中性粒细胞外细胞陷阱(NETs),DNase I可能有望缓解视网膜静脉阻塞(RVO)的症状和进展。然而,该研究还承认存在某些限制,例如需要进一步研究DNase I的最佳剂量以及其在RVO背景下的具体作用机制。此外,该研究强调了在未来研究中纳入更大规模和更多样化的患者群体以提高结果的有效性的重要性。
总之,该研究将DNase I定位为治疗RVO的有前途候选药物,特别是由于其在减少NETs形成和减轻视网膜血栓持续时间方面的有效性。这代表了治疗RVO的治疗方法的潜在转变,从仅仅管理并发症到解决该疾病的主要根本原因之一。进一步的研究对于全面了解DNase I的作用以及为其融入RVO的临床治疗奠定基础至关重要。
Reference: Deng G, Zou X, Liu Z, Ren H, Li Y, Chen B, et al. The protective effect of DNase I in retinal vein occlusion. Biomol Biomed [Internet]. 2023 Oct. 12 [cited 2024 Jan. 24];. Available from: https://www.bjbms.org/ojs/index.php/bjbms/article/view/9780
Editor: Ermina Vukalic
Leave a Reply