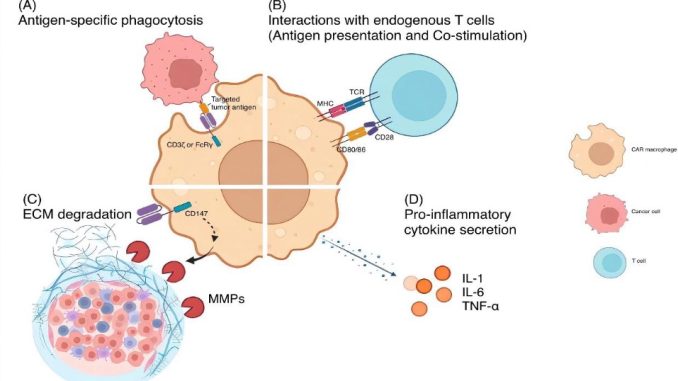
In the ongoing quest to combat cancer, chimeric antigen receptor (CAR) T therapy has emerged as a promising treatment, revolutionizing the way we enhance a patient’s immune cells within the laboratory to escalate their assault on cancerous tumors. However, this innovative therapy encounters significant resistance when deployed against solid tumors due to the dauntingly hostile environment these tumors manufacture within the organs.
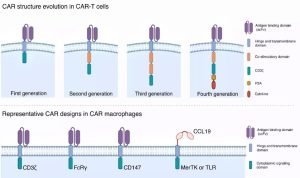
Addressing this challenge, another therapeutic contender enters the arena: CAR macrophage therapy. This novel approach ingeniously modifies immune cells known as macrophages with CARs, displaying a potent potential specifically tailored for solid tumors. In this review, we navigate through the labyrinth of CAR-T and CAR macrophage therapies, probing their structural intricacies, their operational tactics against cancer, and the formidable barriers solid tumors present, all the while drawing invaluable lessons from clinical trial experiences.
What sets CAR macrophage therapy apart, as this review suggests, are its two distinct advantages in combatting solid tumors: it deploys a broader spectrum of tumor-fighting strategies and boasts superior access to the very heart of tumor sites. Notwithstanding, it shares with CAR T therapy certain limitations, particularly the challenge of identifying suitable targets on the elusive tumor cells themselves. This review serves not only to juxtapose the two modalities but also to contemplate how they might learn from each other to enhance their effectiveness against solid tumors.
Moreover, we highlight the critical questions that must be addressed by future research. The imperative to identify novel targets for these therapies is underscored, aiming to amplify their safety and efficacy. Additionally, the urgent need for more extensive clinical trials with a laser focus on solid tumors is emphasized, in order to deepen our comprehension of the advantages and potential risks associated with both CAR-T and CAR macrophage therapies.
The cross-pollination of insights and therapeutic strategies between CAR-T and CAR macrophage therapies offers a tantalizing glimpse into the future of oncological treatment. As this field evolves, it becomes increasingly clear that each therapy has much to teach the other, potentially paving the way for a new era in the management and eradication of solid tumors. Through a synergy of innovative research and clinical prowess, we stand on the cusp of transformative advances in cancer therapy.
The translation of preceeding text in Chinese:
在持续对抗癌症的努力中,一种被称为嵌合抗原受体(CAR)T细胞疗法的前景充满希望的治疗方法涌现了,它通过在实验室中增强患者的免疫细胞,以提高它们对抗肿瘤的能力。然而,针对实体瘤,如器官中发现的那些肿瘤,这种疗法面临着挑战,因为这些肿瘤创造了一个严酷的环境。另一种方法,CAR巨噬细胞疗法,通过对称为巨噬细胞的免疫细胞进行工程改造,使它们带有CARs,显示出治疗实体瘤的潜力。
在这篇综述中,我们探讨了CAR-T和CAR巨噬细胞疗法之间的差异和相似之处。我们研究了它们的结构,它们如何对抗肿瘤,以及它们在对抗实体瘤时遇到的挑战,以及从临床试验中获得的洞察。理解这些方面有助于改进这两种疗法。
我们的综述表明,对于实体瘤,CAR巨噬细胞疗法可能有两个关键优势。它似乎提供了更广泛的抗肿瘤方法,并且能更好地到达肿瘤部位。然而,它面临着与CAR T疗法类似的问题,例如缺乏肿瘤细胞上的适合靶点。我们还讨论了这些疗法如何能够互相学习,以便在治疗实体瘤方面更加有效。
此外,我们提出了未来研究的重要问题,例如为这些疗法确定新的靶点,使它们更加安全和有效。我们还强调了进行更多关注实体瘤的广泛临床试验的必要性,以便深入了解CAR-T和CAR巨噬细胞疗法的利弊。CAR-T和CAR巨噬细胞疗法之间的见解和治疗策略的相互渗透,为肿瘤学治疗的未来提供了一瞥。随着这一领域的发展,越来越清楚的是,每种疗法都有很多可以教给对方的东西,这可能为管理和根除实体瘤开辟了一个新时代。通过创新研究和临床能力的协同作用,我们站在了癌症治疗变革性进展的前沿。
Reference: Chen K, Liu M- ling, Wang J- cheng, Fang S. CAR-macrophage versus CAR-T for solid tumors: The race between a rising star and a superstar. Biomol Biomed [Internet]. 2023Oct.24 [cited 2023Nov.2];. Available from: https://www.bjbms.org/ojs/index.php/bjbms/article/view/9675
Editor: Ermina Vukalic
Leave a Reply