By Semir Vranic, MD, PhD
Bosnian Journal of Basic Medical Sciences
Editor in Chief
Food and Drug Administration (FDA) has just granted accelerated approval to sacituzumab govitecan-hziy (TRODELVY, Immunomedics, Inc.) for the patients with metastatic triple-negative breast cancer (TNBC) that were treated with at least two prior therapies for their metastatic disease.
The FDA decision was based on the efficacy of sacituzumab govitecan-hziy that was demonstrated in the IMMU-132-01 (NCT 01631552) clinical trial (Bardia et al. N Engl J Med 2019;380:741-51). It was a multicenter, single-arm, trial that enrolled 108 patients with metastatic TNBC. The patients received sacituzumab govitecan-hziy 10 mg/kg intravenously on days 1 and 8 every 21days. The tumor imaging was obtained every 8 weeks, and patients were treated until disease progression or intolerance to therapy.
The primary outcome measures were overall response rate (ORR) and response duration. The ORR was 33.3% (95% CI: 24.6-43.1) while the median response duration was 7.7 months (95% CI: 4.9-10.8) (Bardia et al. N Engl J Med 2019;380:741-51).
The recommended sacituzumab govitecan-hziy dose is 10 mg/kg administered by intravenous infusion administered on days 1 and 8 every 21 days until disease progression or unacceptable toxicity (Bardia et al. N Engl J Med 2019;380:741-51).
Sacituzumab govitecan-hziy was approved under accelerated approval, which was based on ORR and response duration measures. However, continued approval for this indication may be contingent upon further verification of clinical benefits in confirmatory clinical trials.
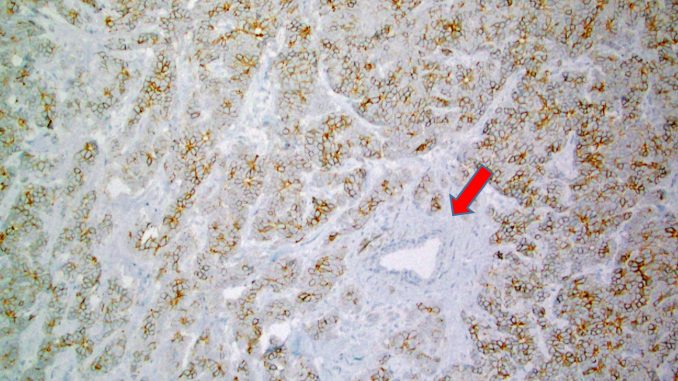
Notably, Trodelvy is the first antibody-drug conjugate (ADC) that has been approved by the FDA for relapsed or refractory metastatic TNBCs and is also the first FDA-approved anti-Trop-2 ADC. Trodelvy contains irinotecan metabolite, SN-38 that is conjugated to a humanized anti-TROP-2 antibody (sacituzumab govitecan) (Goldenberg et al. Oncotarget 2018). Trop-2 (=trophoblast cell-surface antigen 2) has originally been described in trophoblasts but subsequently, Trop-2 expression has been described in various cancers including a common expression in breast cancer (Figure 1). Many normal tissues lack or show low Trop-2 protein expression (Figure 1 shows a normal breast duct that is spared of Trop-2 positivity). Trop-2 protein contains a 274-amino-acid extracellular EGF-like repeat portion that contains three domains, a cysteine-rich domain, a thyroglobulin type-1 domain, and a cysteine-poor domain (Trop-2 is nicely reviewed in Goldenberg et al. Oncotarget 2018). Trop-2 signaling includes various cellular interactions including those with IGF-1, claudin-1, claudin-7, cyclin D1, and Protein kinase C.

Another component of Trodelvy is SN-38, an irinotecan metabolite. Irinotecan is a well-known anti-neoplastic drug that has been used for the treatment of metastatic colorectal cancer. The key molecular target of irinotecan is topoisomerase I, which is frequently overexpressed in breast cancer. Irinotecan reversibly stabilizes the topoisomerase I cleavable complex, resulting in single-strand DNA breaks and inhibition of DNA religation (Fujita et al. World J Gastroenterol 2015).
Notably, very recent data indicate a promising therapeutic activity of sacituzumab govitecan-hziy in patients with heavily pretreated estrogen receptor (ER)+ and HER2- metastatic breast cancers. A new Phase III clinical trial (TROPiCS-02 study, NCT03901339), evaluating sacituzumab govitecan-hziy versus standard treatment in ER+/HER2- MBC has just been initiated (Rugo et al. Future Oncol 2020;16:705-715).
Leave a Reply