In a recent study conducted by the Institute of Anatomy, Faculty of Medicine, University of Ljubljana, and in collaboration with the Department of Animal Science, Biotechnical Faculty, University of Ljubljana, and the Laboratory of Biomathematics at the Institute of Physiology of the Czech Academy of Sciences, researchers have provided new insights into the detrimental effects of Type 1 diabetes mellitus (T1DM) on skeletal muscle structure and capillary networks. Utilizing state-of-the-art 3D imaging technology, this comprehensive study marks a significant leap in understanding the multifaceted impact of T1DM on the body’s muscular system.
Diabetes mellitus disrupts the regulation of glucose levels, leading to high blood sugar and a myriad of related health issues. T1DM, characterized by the immune-mediated destruction of insulin-producing pancreatic β cells, has profound effects on various organs, especially skeletal muscles, which play a crucial role in glucose uptake and regulation. This study aimed to explore the structural and functional adaptations of skeletal muscles to the metabolic disturbances caused by T1DM.
The Hidden Changes in Muscle and Blood Vessels
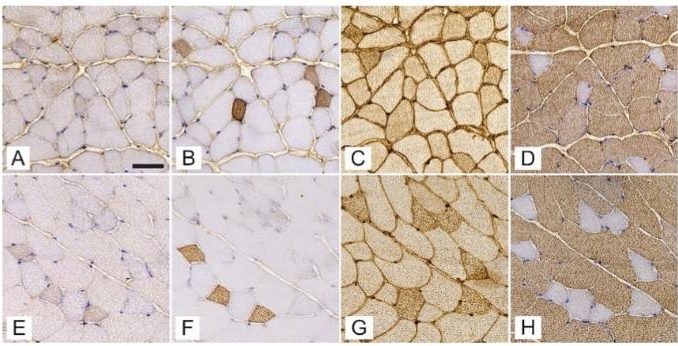
Conducted on female C57BL/6J-OlaHsd mice using a streptozotocin (STZ)-induced model to simulate T1DM, the research focused on critical muscles like the soleus, gluteus maximus, and gastrocnemius. Researchers meticulously analyzed the expression of myosin heavy chain (MyHC) isoforms and the intricacies of the 3D capillary network. “Our study provides a deeper understanding of how Type 1 diabetes not only affects muscle fiber composition but also significantly alters the capillary networks that are essential for muscle health,” explained Nejc Umek, the study’s lead author.
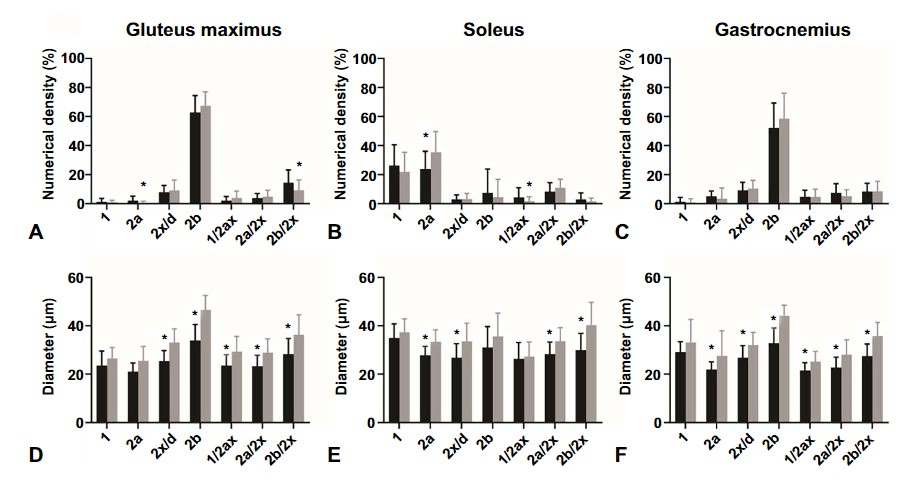
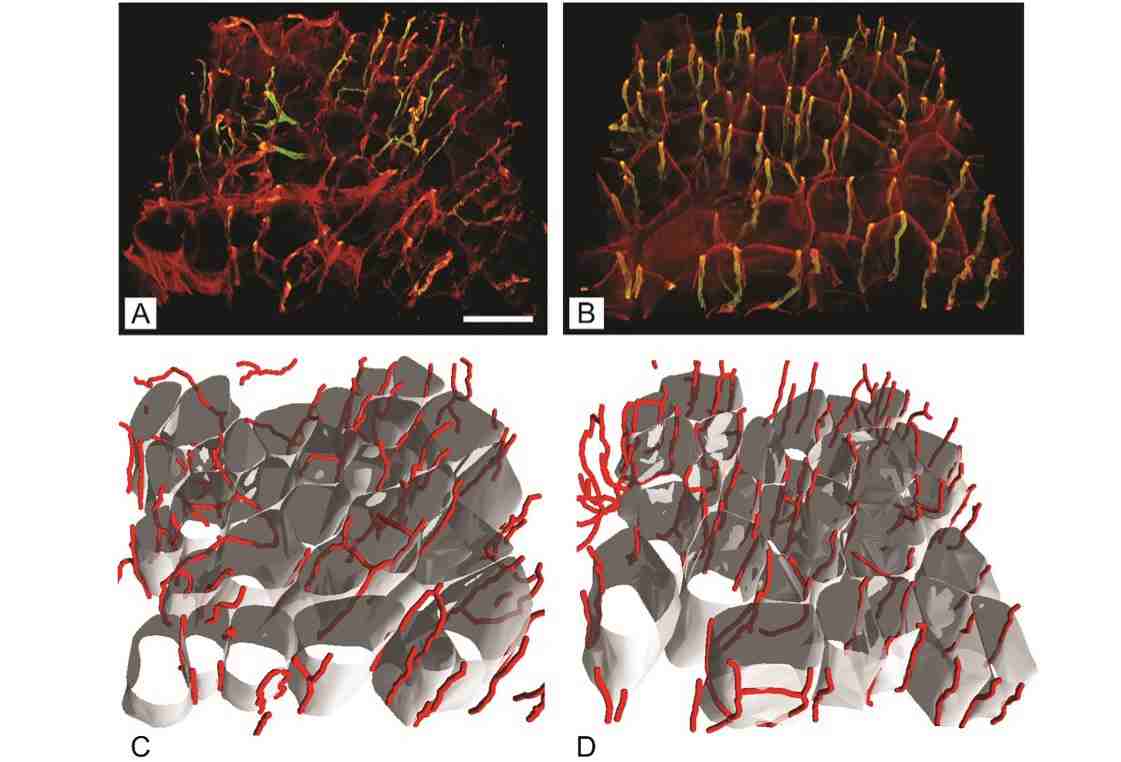
The research revealed that, despite the composition of fast-twitch type 2b fibers remaining consistent, notable differences were observed in the diabetic mice’s soleus muscle, which showed a reduced proportion of type 2a fibers and diminished fiber diameters across all muscles analyzed. Additionally, an intriguing increase in capillary length per muscle volume was discovered in the gluteus maximus of diabetic mice, suggesting an adaptive mechanism to counterbalance muscle fiber atrophy induced by diabetes.
Methodological Advances and Key Discoveries
The study utilized female mice, addressing a gap in diabetes research that often overlooks gender differences in disease progression and response to treatment. Through a single intraperitoneal administration of STZ, researchers successfully induced T1DM, confirmed by significantly elevated fasting glucose levels. This model allowed for an in-depth examination of diabetes-induced changes in a controlled environment.
By employing antibodies specific to different MyHC isoforms and cutting-edge 3D imaging, the team was able to precisely quantify changes in muscle fiber types and the capillary network. “The advanced 3D imaging techniques we used represent a significant improvement over traditional 2D analyses, offering a more detailed and accurate depiction of the capillary network changes in diabetic muscle tissue,” stated Erika Cvetko, the study’s senior author.
Implications for Diabetes Management and Future Directions
The findings from this collaborative research effort highlight the necessity for comprehensive diabetes management plans that encompass not only glucose regulation but also the preservation of muscle structure and function. “Understanding the specific alterations in muscle tissue due to Type 1 diabetes paves the way for developing targeted therapies that could significantly improve patient outcomes,” Cvetko added.
The study’s revelations about the increased capillary length per muscle volume in diabetic mice underscore the body’s potential compensatory responses to the structural changes induced by diabetes. These insights are crucial for designing interventions that aim to mitigate muscle deterioration and enhance overall diabetes care.
This novel study contributes significantly to the body of knowledge on diabetes and its systemic effects, particularly on skeletal muscle health. By highlighting the critical role of maintaining muscle integrity and vascular supply in the management of T1DM, the research opens new avenues for therapeutic strategies and underscores the importance of multidisciplinary approaches in tackling this complex disease.
The translation of the preceding English text in Slovenian:
V nedavni študiji, ki so jo izvedli na Inštitutu za anatomijo, Medicinski fakulteti Univerze v Ljubljani, v sodelovanju z Oddelkom za živalsko znanost, Biotehniško fakulteto Univerze v Ljubljani, in Laboratorijem za biomatematiko na Inštitutu za fiziologijo Češke akademije znanosti, so raziskovalci ponudili nove vpoglede v škodljive učinke sladkorne bolezni tipa 1 (T1DM) na strukturo skeletnih mišic in kapilarnih mrež. Z uporabo najsodobnejše tehnologije 3D slikanja ta obsežna študija predstavlja pomemben preskok v razumevanju večplastnega vpliva T1DM na mišični sistem telesa.
Sladkorna bolezen moti uravnavanje ravni glukoze, kar vodi v visok krvni sladkor in vrsto povezanih zdravstvenih težav. T1DM, za katerega je značilno imunsko posredovano uničenje insulinoproizvajajočih β celic trebušne slinavke, ima globok učinek na različne organe, še posebej na skeletne mišice, ki igrajo ključno vlogo pri prevzemu in uravnavanju glukoze. Ta študija je bila namenjena osvetlitvi strukturnih in funkcionalnih prilagoditev skeletnih mišic na metabolične motnje, ki jih povzroča T1DM.
Skrite spremembe v mišicah in krvnih žilah
Izvedena na samicah miši C57BL/6J-OlaHsd z uporabo modela, ki ga je povzročil streptozotocin (STZ), za simulacijo T1DM, se je raziskava osredotočila na ključne mišice, kot so soleus, gluteus maximus in gastrocnemius. Raziskovalci so natančno analizirali izražanje izoform težke verige miozina (MyHC) in zapletenosti 3D kapilarnega omrežja. “Naša študija ponuja globlje razumevanje, kako sladkorna bolezen tipa 1 ne vpliva le na sestavo mišičnih vlaken, ampak tudi bistveno spreminja kapilarna omrežja, ki so ključna za zdravje mišic,” je pojasnil Nejc Umek, vodilni avtor študije.
Raziskava je razkrila, da kljub konstantni sestavi hitrih vlaken tipa 2b, opazne razlike v soleus mišici diabetičnih miši, ki so pokazale zmanjšan delež vlaken tipa 2a in zmanjšane premer vlaken v vseh analiziranih mišicah. Poleg tega je bilo odkrito zanimivo povečanje dolžine kapilar na volumen mišice v gluteus maximus diabetičnih miši, kar kaže na prilagoditveni mehanizem za uravnoteženje atrofije mišičnih vlaken, ki jo povzroča diabetes.
Metodološki napredki in ključna odkritja
Študija je uporabljala samice miši, naslavljajoč vrzel v raziskavah diabetesa, ki pogosto spregleda razlike med spoloma v napredovanju bolezni in odzivu na zdravljenje. Z enkratno intraperitonealno uporabo STZ so raziskovalci uspešno povzročili T1DM, kar je bilo potrjeno z znatno povišanimi nivoji postnega glukoze. Ta model je omogočil poglobljen pregled sprememb, ki jih povzroča diabetes, v kontroliranem okolju.
Z uporabo protiteles, specifičnih za različne izoforme MyHC, in napredno 3D slikanje, je ekipi uspelo natančno kvantificirati spremembe v tipih mišičnih vlaken in kapilarnem omrežju. “Napredne tehnike 3D slikanja, ki smo jih uporabili, predstavljajo pomemben napredek v primerjavi s tradicionalnimi 2D analizami in nudijo bolj podroben in natančen prikaz sprememb kapilarnega omrežja v diabetičnem mišičnem tkivu,” je povedala Erika Cvetko, višja avtorica študije.
Posledice za obvladovanje diabetesa in prihodnje usmeritve
Ugotovitve iz tega sodelovalnega raziskovalnega prizadevanja poudarjajo potrebo po celovitih načrtih obvladovanja diabetesa, ki ne zajemajo le uravnavanja glukoze, ampak tudi ohranjanje strukture in funkcije mišic. “Razumevanje specifičnih sprememb v mišičnem tkivu zaradi sladkorne bolezni tipa 1 odpira pot za razvoj ciljno usmerjenih terapij, ki bi lahko znatno izboljšale izide za paciente,” je dodala Cvetko.
Razkritja študije o povečani dolžini kapilar na volumen mišice pri diabetičnih miših poudarjajo možne kompenzacijske odzive telesa na strukturne spremembe, ki jih povzroča diabetes. Ta vpogled je ključen za načrtovanje intervencij, ki ciljajo na zmanjšanje poslabšanja mišic in izboljšanje celostne oskrbe diabetesa.
Ta novatorska študija pomembno prispeva k znanju o diabetesu in njegovih sistemskih učinkih, še posebej na zdravje skeletnih mišic. S poudarjanjem ključne vloge ohranjanja integritete mišic in vaskularne oskrbe pri obvladovanju T1DM raziskava odpira nove poti za terapevtske strategije in poudarja pomembnost multidisciplinarnih pristopov pri spopadanju s to kompleksno boleznijo.
Reference: Umek N, Pušnik L, Ugwoke CK, Šink Žiga, Horvat S, Janáček J, et al. Skeletal muscle myosin heavy chain expression and 3D capillary network changes in streptozotocin-induced diabetic female mice. Biomol Biomed [Internet]. 2023 Oct. 30 [cited 2024 Feb. 1];. Available from: https://www.bjbms.org/ojs/index.php/bjbms/article/view/9843
Editor: Ermina Vukalic
Leave a Reply