Research by experts from Ningbo Eye Hospital and Hwa Mei Hospital at the University of Chinese Academy of Sciences provides insight into a critical issue facing surgical patients: Post-Operative Cognitive Dysfunction (POCD).
Post-Operative Cognitive Dysfunction, commonly abbreviated as POCD, is a neurological complication that some patients experience after undergoing surgical procedures that require anesthesia. It manifests as a noticeable decline in cognitive abilities, specifically impacting areas like learning, memory, concentration, and even causing changes in personality. According to this scholarly article, traditional treatments for POCD mainly focus on regulating nutrition and fluid balance, maintaining electrolytes, and providing psychological support. Some patients are also given neurotrophic drugs to support nerve growth and health.
The Issue at Hand: Cognitive Risks After Anesthesia
Anesthesia is a medical practice involving the administration of drugs to block sensation, making patients unconscious or insensitive to pain during surgical or diagnostic procedures. The practice encompasses various forms: general anesthesia for whole-body unconsciousness, regional anesthesia for blocking sensation in a specific body area, and local anesthesia for numbing a particular site. Anesthetic agents act on the central nervous system by targeting specific neurotransmitter receptors to inhibit nerve impulses, which alters perception, motor function, and consciousness. While essential for pain management in surgeries, anesthesia is not without risks.
Sevoflurane, a widely-used anesthetic, has been identified as a significant factor contributing to postoperative cognitive dysfunction (POCD). Belonging to the fluorinated ether class of anesthetics, sevoflurane is commonly used to induce and maintain general anesthesia. Its rapid onset and offset make it suitable for a variety of surgical procedures. While generally considered safe and effective, sevoflurane has been linked to certain neurocognitive deficits, including POCD. Research indicates that exposure to sevoflurane can cause pathological changes in the brain, such as amyloid-beta (Aβ) deposition and fibrin tangles in the hippocampus, which are associated with cognitive impairment. Alarmingly, these effects have also been observed in the offspring of rats exposed to the drug during pregnancy.
Given these findings, mitigating the risk factors or symptoms of POCD, especially those induced by sevoflurane, has become an urgent priority for clinicians and researchers alike.
Enter Resveratrol: The Natural Brain Protector
Resveratrol, often abbreviated as RES, is a bioactive compound found in certain foods like peanuts, grapes, and blueberries. It has the ability to cross the blood-brain barrier, meaning it can directly interact with the central nervous system (CNS). This compound has garnered attention for its multi-faceted biological effects, including its anti-inflammatory, antioxidant, and anti-apoptotic (cell-death preventing) properties.
In the context of neuroscience and medicine, RES is a known agonist for Silent Information Regulator 1 (SIRT1), a protein that has various neuroprotective roles. By binding to and activating SIRT1, RES can have a protective effect on the brain. For example, it aids in regulating the growth and differentiation of neurons, can prevent cell death, and has shown promise in combating conditions like Alzheimer’s disease. Moreover, RES has demonstrated its capability to modulate pathways like the cAMP/AMPK/SIRT1, which is particularly relevant in providing neuroprotective effects in stroke scenarios. It can also regulate inflammation, offering potential benefits for aging and age-related diseases.
According to this scholarly article, previous research has shown that RES could improve cognitive impairments in newborn mice that were exposed to sevoflurane (SEV). However, this effect had not yet been studied in adult animal models. The aim of this study was to fill that knowledge gap. It explored the role of the SIRT1/RhoA signaling pathway in the neurotoxic effects caused by long-term exposure to SEV. Moreover, the study looked into how administering RES before and after surgery could potentially mitigate cognitive impairment in adult rats exposed to SEV.
Experimental Procedures and Protocols
The study used 76 adult male rats of a specific kind called Sprague-Dawley (SD) rats. SD rats are a common type of lab rat that scientists use for research. They’re chosen because they’re easy to handle and have a well-understood biology, which makes them good for studying how diseases work or how different treatments might work. Think of them as the “standard model” when scientists need to test something in a living creature before they can consider trying it in humans. These rats weighed between 280-320 grams and were 8-10 weeks old. They were kept in a special, controlled environment that met ethical guidelines for animal research.
What is and How the SEV Model Works?
The “SEV model” is a way to study the effects of the sevoflurane (SEV) on rats. In this experiment, rats are placed in special boxes and breathe in a mixture of 3% sevoflurane and 40% oxygen for 6 hours. During this time, scientists carefully watch the rats to make sure they’re breathing normally and aren’t showing any signs of stress. After the 6 hours, the rats are allowed to wake up and are returned to their usual living spaces. This model helps researchers understand how long-term exposure to this anesthesia gas might affect the brain and other parts of the body.
As the researchers were interested in resveratrol (RES), they dissolved it in a chemical called dimethyl sulfoxide (DMSO) and then diluted it with saline (saltwater) so it could be easily injected into the rats. DMSO is a chemical that’s often used as a “carrier” to help other substances get where they need to go. They injected it into the rats’ body cavities at three different doses, either a day before or an hour after the SEV exposure.
Types of Tests Conducted on Rats
Short-term Neurological Test: Two days after the SEV exposure, researchers used the Negative Geotaxis Test to check a rat’s balance and coordination. In this test, a rat is placed head-down on a sloping board. The goal is to see how quickly the rat can turn itself right-side-up. This helps gauge how well the rat’s brain and muscles are coordinating.
Long-term Neurological Test: Two weeks after the SEV exposure, the researchers placed the rats on a rotating rod to see how long they could stay on it. This is known as the Rotarod Test. The Rotarod Test is essentially a treadmill challenge for rats. In this test, a rat is put on a spinning rod, and the goal is to measure how long the rat can stay on it without falling off. This helps researchers gauge the rat’s balance, coordination, and motor skills. It serves as a way to check for long-term neurological issues in the rat, particularly after being exposed to certain treatments or conditions.
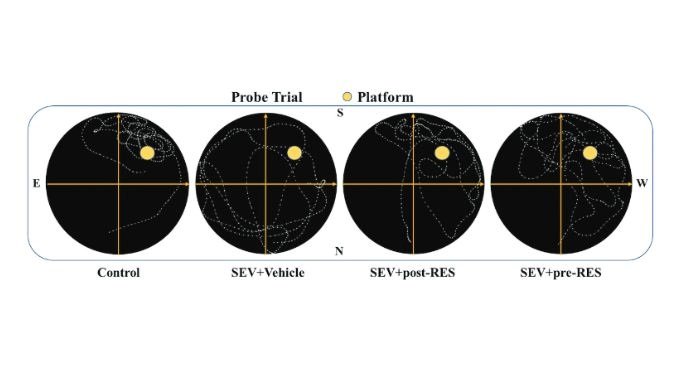
Memory and Learning Test: About three weeks after the SEV exposure, the researchers used a test known as the Morris Water Maze. This test evaluates how well rats can remember and find a hidden platform in a pool of water. In the Morris Water Maze, a rat is put into a pool with a submerged platform that it can’t see. The rat has to swim around until it finds this hidden platform and climbs onto it. Researchers keep track of how fast the rat locates the platform and how well it remembers the platform’s location in subsequent tests. This provides insights into the rat’s memory and learning abilities.
Checking Protein Levels in the Brain
Finally, the researchers conducted a biochemical analysis using Western Blotting. This technique measures the levels of specific proteins in the rats’ brain tissues, particularly those involved in cell death and signaling pathways, allowing for a detailed understanding of how SEV and RES affect the brain at the molecular level.
Think of Western Blotting like a blood test that’s designed to detect specific markers or indicators of a condition. Just as a blood test can identify the levels of cholesterol or glucose to diagnose or monitor certain diseases, Western Blotting identifies specific proteins to understand cellular processes.
In this study, after exposing the rats to SEV and/or resveratrol, researchers extracted small samples of the rats’ brain tissue and separated them into individual components. Using Western Blotting, they were able to identify specific proteins that serve as markers for various processes, like cell death or survival. By doing so, they gained a deeper understanding of the molecular events taking place inside the rat’s brain cells.
Statistical Tools Used and Results of the Study
The researchers used specialized statistical tests, like one-way and two-way ANOVA, to make sense of the data. In simple terms, these tests act like advanced calculators to figure out if the changes seen in rats were because of the treatments or just random chance. A key value is “p < 0.05,” meaning that results below this number are likely not random and are statistically significant.
In the study, 76 rats were tested, and all survived the different treatments. Three doses of a substance called resveratrol were given, and the medium dose worked the best. After being exposed to sevoflurane (SEV), the rats had problems with orientation and weight loss, but resveratrol helped them recover. Tests also showed that resveratrol improved the rats’ balance, motor skills, learning, and memory. At the molecular level, resveratrol acted like a “first-aid kit,” reversing changes caused by SEV exposure. Overall, resveratrol seems promising for countering SEV’s negative effects on rats’ behavior and cellular health.
The study aimed to assess whether resveratrol could mitigate brain damage induced by SEV, a common anesthetic. SEV exposure resulted in brain cell damage and impaired learning and memory in rats. Resveratrol effectively prevented these issues, particularly when administered preemptively before SEV exposure. This implies that employing resveratrol as a preventive measure could prove especially advantageous for individuals susceptible to postoperative cognitive dysfunction (POCD). Nevertheless, while the study yielded valuable insights, there remain avenues for further investigation. For instance, given the study’s exclusive focus on male rats, it’s imperative to broaden the research scope to encompass female subjects.
In summary, resveratrol demonstrates significant potential in shielding against brain and cognitive impairments arising from SEV exposure in rats. Administering it prior to exposure appears to amplify its protective efficacy, likely through the SIRT1/RhoA pathway.
The translation of the preceding English text in Chinese:
由宁波眼科医院和中国科学院大学华美医院的专家进行的研究为外科病人面临的一个关键问题提供了见解:术后认知功能障碍(POCD)。
术后认知功能障碍,通常简称为POCD,是一些病人在接受需要麻醉的手术程序后经历的一种神经并发症。它表现为明显的认知能力下降,特别是影响学习、记忆、注意力,甚至导致性格变化。根据这篇学术文章,POCD的传统治疗主要集中在调节营养和液体平衡、维持电解质平衡和提供心理支持。一些病人还被给予神经营养药物来支持神经生长和健康。
手头的问题:麻醉后的认知风险
麻醉是一种涉及药物管理的医疗实践,以阻止感觉,使病人在外科或诊断程序中失去知觉或对疼痛不敏感。这个实践包括各种形式:全身麻醉使整个身体失去知觉,局部麻醉阻止特定身体区域的感觉,局部麻醉使特定部位麻木。麻醉药物通过针对特定的神经递质受体来抑制神经冲动,从而改变知觉、运动功能和意识。虽然对手术中的疼痛管理至关重要,但麻醉并非没有风险。
氟烷,一种广泛使用的麻醉药,被认为是术后认知功能障碍(POCD)的一个重要因素。氟烷属于氟化醚类麻醉药,常用于诱导和维持全身麻醉。其快速起效和停效使其适用于各种外科手术。虽然通常被认为是安全和有效的,但氟烷与某些神经认知缺陷有关,包括POCD。研究表明,暴露于氟烷会导致大脑发生病理性变化,如海马中的淀粉样β(Aβ)沉积和纤维蛋白缠结,这与认知障碍有关。令人担忧的是,这些效应也被观察到在怀孕期间暴露于该药物的大鼠的后代中。
鉴于这些发现,减轻POCD的风险因素或症状,尤其是那些由氟烷引起的,已经成为临床医生和研究者的紧迫任务。
Resveratrol:天然的脑保护剂
白藜芦醇,常简称为RES,是一种存在于某些食物中的生物活性化合物,如花生、葡萄和蓝莓。它能够穿越血脑屏障,意味着它可以直接与中枢神经系统(CNS)互动。这种化合物因其多种生物学效应而受到关注,包括其抗炎、抗氧化和抗凋亡(预防细胞死亡)的特性。
在神经科学和医学的背景下,RES是已知的寂静信息调节蛋白1(SIRT1)的激动剂,SIRT1是一种具有各种神经保护作用的蛋白质。通过与SIRT1结合并激活,RES可以对大脑产生保护作用。例如,它有助于调节神经元的生长和分化,可以预防细胞死亡,并已经显示出对抗诸如阿尔茨海默病这样的疾病的希望。此外,RES已经证明了它调节cAMP/AMPK/SIRT1等途径的能力,这在提供中风情境中的神经保护效应方面尤为相关。它还可以调节炎症,为衰老和与年龄相关的疾病提供潜在的益处。
根据这篇学术文章,以前的研究已经显示,RES可以改善暴露于氟烷(SEV)的新生小鼠的认知障碍。然而,这种效应尚未在成年动物模型中进行研究。这项研究的目的是填补这一知识空白。它探讨了SIRT1/RhoA信号通路在长期暴露于SEV引起的神经毒性效应中的作用。此外,这项研究还探讨了在手术前后给予RES如何可能减轻暴露于SEV的成年大鼠的认知障碍。
实验程序和协议
这项研究使用了76只特定种类的成年雄性大鼠,称为斯普拉格-道利(SD)大鼠。SD大鼠是科学家用于研究的常见类型的实验鼠。它们被选择是因为它们容易处理,并且有一个被很好理解的生物学,这使它们适合研究疾病是如何工作的或不同的治疗可能是如何工作的。当科学家需要在一个生物体中测试某物之前,可以考虑尝试它在人类中,可以把它们看作是科学家的“标准模型”。这些大鼠体重在280-320克之间,年龄在8-10周之间。它们被放在一个特殊的,受控的环境中,满足动物研究的伦理指南。
什么是SEV模型,它是如何工作的?
“SEV模型”是研究氟烷(SEV)对大鼠的影响的一种方法。在这个实验中,大鼠被放在特殊的盒子里,呼吸3%的氟烷和40%的氧气混合物6小时。在此期间,科学家们仔细观察大鼠,以确保它们正常呼吸,没有显示任何压力的迹象。6小时后,允许大鼠醒来,并返回到它们通常的居住空间。这个模型帮助研究者了解长期暴露于这种麻醉气体可能如何影响大脑和身体的其他部分。
由于研究者对白藜芦醇(RES)感兴趣,他们将其溶解在称为二甲基亚砜(DMSO)的化学物质中,然后用生理盐水稀释,以便可以容易地注射到大鼠体内。DMSO是一种经常被用作”载体”的化学物质,以帮助其他物质到达它们需要去的地方。他们在SEV暴露前一天或暴露后一小时将其注射到大鼠的体腔中,共三种不同的剂量。
对大鼠进行的测试类型
短期神经测试:SEV暴露后两天,研究者使用负地向性测试来检查大鼠的平衡和协调。在这个测试中,一只大鼠被放在一个倾斜的板上,头朝下。目标是看大鼠多快能把自己翻过来。这有助于衡量大鼠的大脑和肌肉是如何协调的。
长期神经测试:SEV暴露后两周,研究者将大鼠放在一个旋转的杆上,看它们能在上面停留多长时间。这被称为旋转杆测试。旋转杆测试实际上是大鼠的跑步机挑战。在这个测试中,一只大鼠被放在一个旋转的杆上,目标是测量大鼠能在上面停留多长时间而不掉下来。这帮助研究者衡量大鼠的平衡、协调和运动技能。它是一种检查大鼠是否存在长期神经问题的方法,特别是在暴露于某些治疗或条件之后。
记忆和学习测试:SEV暴露后约三周,研究者使用了一个称为莫里斯水迷宫的测试。这个测试评估大鼠如何记住和找到水池中的一个隐藏的平台。在莫里斯水迷宫中,一只大鼠被放入一个有一个它看不到的浸没的平台的水池中。大鼠必须游泳,直到它找到这个隐藏的平台并爬到上面。研究者记录大鼠找到平台的速度,以及它在随后的测试中对平台位置的记忆情况。这为大鼠的记忆和学习能力提供了见解。
检查大脑中的蛋白质水平
最后,研究者使用Western Blotting进行生化分析。这种技术测量大鼠大脑组织中特定蛋白质的水平,特别是那些涉及细胞死亡和信号通路的蛋白质,允许详细了解SEV和RES如何影响大脑在分子水平上。
可以把Western Blotting看作是一种设计用来检测某种条件的特定标记或指标的血液测试。就像血液测试可以识别胆固醇或葡萄糖的水平来诊断或监测某些疾病,Western Blotting识别特定的蛋白质以了解细胞过程。
在这项研究中,在暴露大鼠于SEV和/或白藜芦醇后,研
究者提取了大鼠的大脑组织的小样本,并将它们分离成单独的组分。使用Western Blotting,他们能够识别那些作为各种过程的标记的特定蛋白质,如细胞死亡或生存。通过这样做,他们对大鼠大脑细胞内发生的分子事件有了更深入的了解。
使用的统计工具和研究结果
研究者使用了专门的统计测试,如单向和双向ANOVA,来理解数据。简单来说,这些测试就像高级计算器,用来判断在大鼠身上看到的变化是因为治疗还是纯粹是随机的。一个关键值是”p < 0.05″,这意味着低于这个数字的结果可能不是随机的,是统计上显著的。
在这项研究中,76只大鼠被测试,所有的大鼠都经受了不同的治疗。给予了三种剂量的白藜芦醇,中等剂量效果最好。暴露于氟烷(SEV)后,大鼠出现了方向和体重减轻的问题,但白藜芦醇帮助它们恢复。测试还显示,白藜芦醇提高了大鼠的平衡、运动技能、学习和记忆。在分子水平上,白藜芦醇像一个”急救包”,逆转了SEV暴露引起的变化。总的来说,白藜芦醇对抵消SEV对大鼠行为和细胞健康的负面影响似乎很有希望。
这项研究旨在评估白藜芦醇是否可以减轻SEV引起的大脑损伤,SEV是一种常见的麻醉药。SEV暴露导致大鼠的大脑细胞损伤和学习和记忆受损。白藜芦醇有效地预防了这些问题,特别是在SEV暴露之前预先给予。这意味着,在SEV暴露之前使用白藜芦醇作为预防措施可能对易患术后认知功能障碍(POCD)的个体尤其有益。尽管这项研究提供了宝贵的见解,但仍然有进一步的研究途径。例如,考虑到这项研究只关注雄性大鼠,扩大研究范围以包括女性受试者是必要的。
总之,白藜芦醇显示出显著的潜力,以抵御SEV暴露在大鼠中引起的大脑和认知障碍。在暴露之前给予它似乎可以放大其保护效果,可能通过SIRT1/RhoA途径。
Reference: Zhou Q, Deng Y, Hu X, Xu Y. Resveratrol ameliorates neuronal apoptosis and cognitive impairment by activating the SIRT1/RhoA pathway in rats after anesthesia with sevoflurane. Bosn J of Basic Med Sci [Internet]. 2022Feb.1 [cited 2023Aug.31];22(1):110-7. Available from: https://www.bjbms.org/ojs/index.php/bjbms/article/view/5997
Editor: Ermina Vukalic
Leave a Reply