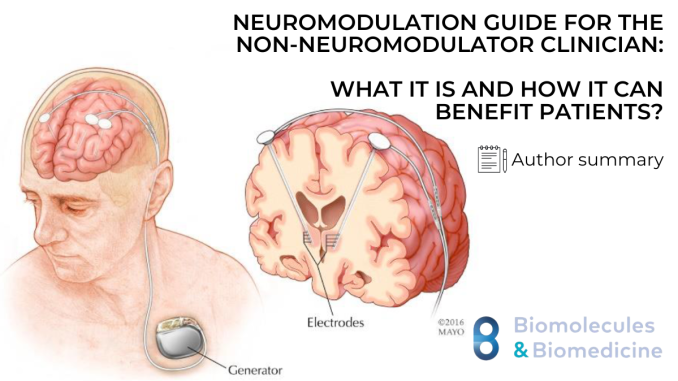
Neuromodulation is increasingly being used in various medical fields to treat both painful and non-painful conditions. Despite its growing application, research on neuromodulation often appears in specialized journals, making it less accessible to general practitioners and specialists outside of pain medicine and neurosurgery. This study provides an overview of implantable neuromodulation devices, discussing their approved uses by the Food and Drug Administration (FDA), off-label applications, and the potential risks and benefits associated with each device.
To gather comprehensive information, the researchers searched PubMed and Medline databases for systematic reviews, meta-analyses, and randomized controlled trials focusing on implantable neuromodulation devices. Out of 106 identified studies, 67 met the criteria for the final review.
The devices covered in this review include deep brain stimulation, spinal cord stimulation, dorsal root ganglion stimulation, peripheral nerve stimulation, vagus nerve stimulation, and carotid sinus stimulation. For each device, the review explores the specific areas of the body they target, their FDA-approved uses, and a comparison of their risks and benefits.
This review aims to inform general clinicians about the potential of implantable neuromodulation devices in treating a wide range of medical conditions. As the use of neuromodulation continues to expand, it is essential for all healthcare providers to have a basic understanding of this innovative treatment approach. Future research will likely focus on exploring non-invasive or non-implantable forms of neuromodulation.
The translation of the preceding English text in Chinese:
神经调控技术在多个医学领域中越来越多地被用于治疗疼痛性和非疼痛性疾病。尽管其应用范围不断扩大,关于神经调控的研究通常发表在专业期刊上,这使得普通医生和非疼痛医学或神经外科的专科医生难以获取相关信息。本研究概述了植入性神经调控设备,讨论了其获得美国食品药品监督管理局(FDA)批准的用途、非标记应用,以及每种设备的潜在风险和益处。
为了收集全面的信息,研究人员在PubMed和Medline数据库中搜索了关于植入性神经调控设备的系统评价、荟萃分析和随机对照试验。在识别出的106项研究中,67项符合最终审查的标准。
本综述涵盖的设备包括深部脑刺激、脊髓刺激、背根神经节刺激、周围神经刺激、迷走神经刺激和颈动脉窦刺激。针对每种设备,综述探讨了它们所针对的特定身体部位、FDA批准的用途,以及它们的风险和益处的比较。
本综述旨在向普通临床医生介绍植入性神经调控设备在治疗各种医疗状况中的潜力。随着神经调控技术的使用不断扩大,所有医疗服务提供者都有必要对这种创新的治疗方法有基本的了解。未来的研究可能会重点探索非侵入性或非植入性形式的神经调控。
Reference:
Chelsey Hoffmann, Jinlan Wang, Rushna P. Ali, Ryan S. D’Souza
Neuromodulation guide for the non-neuromodulator clinician: What it is and how it can benefit patients?;
Biomol Biomed [Internet]. 2024 Aug. 11 [cited 2024 Aug. 19];
Available from: https://www.bjbms.org/ojs/index.php/bjbms/article/view/10967
More news: Blog
Editor: Merima Hadžić
Leave a Reply