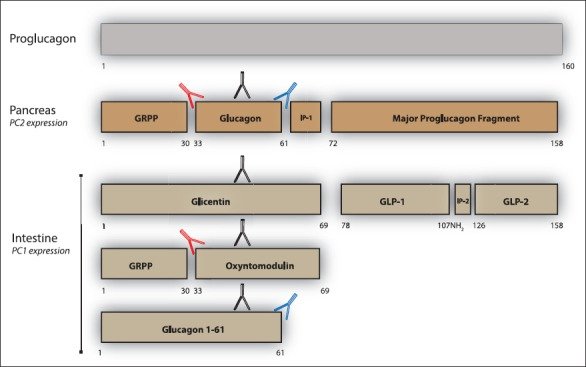
Pancreatic islet α-cell tumours that overexpress proglucagon are typically associated with the glucagonoma syndrome, a rare disease entity characterised by necrolytic migratory erythema, impaired glucose tolerance, thromboembolic complications and psychiatric disturbances. Paraneoplastic phenomena associated with enteric overexpression of proglucagon-derived peptides are less well recognized and include gastrointestinal dysfunction and hyperinsulinaemic hypoglycaemia. The diverse clinical manifestations associated with glucagon-expressing tumours can be explained, in part, by the repertoire of tumorally secreted peptides liberated through differential post-translational processing of tumour-derived proglucagon. Proglucagon-expressing tumours may be divided into two broad biochemical subtypes defined by either secretion of glucagon or GLP-1, GLP-2 and the glucagon-containing peptides, glicentin and oxyntomodulin, due to an islet α-cell or enteroendocrine L-cell pattern of proglucagon processing, respectively. In the current review we provide an updated overview of the clinical presentation of proglucagon-expressing tumours in relation to known physiological actions of proglucagon-derived peptides and suggest that detailed biochemical characterisation of the peptide repertoire secreted from these tumours may provide new opportunities for diagnosis and clinical management.
Learning points:
- Proglucagon-derived peptides exhibit a diverse range of biological activities including critical roles in regulation of glucose and amino acid metabolism, and possibly also affect energy homeostasis as well as cardiovascular and gastrointestinal function.
- Clinical manifestations of proglucagon-expressing tumours exhibit marked phenotypic variation possibly due to heterogeneity of their secreted peptide repertoire.
- Specific and precise biochemical assessment of individuals with proglucagon-expressing tumours may provide opportunities for improved diagnosis and clinical management.
Reference:
Nicolai Jacob Wewer Albrechtsen, Benjamin G. Challis, Ivan Damjanov, Juul Holst Jens. Do glucagonomas always produce glucagon? Bosn J Basic Med Sci. 2016; 16(1): 1–7. doi: 10.17305/bjbms.2015.794
Leave a Reply